Unveiling spatiotemporal dynamics and unconventional functions of the axonal autophagy–lysosomal system
MARINA MIKHAYLOVA (Humboldt-Univerität zu Berlin)

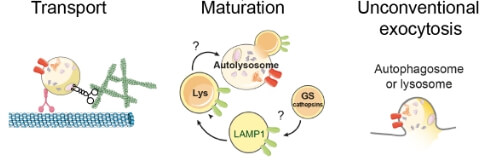
The autophagy-lysosomal system plays a central role in synaptic remodeling, but the exact mechanisms, which allow for tight spatio-temporal control of degradative organelle localization and activity, are still poorly understood. In neurons, secretory-trafficking organelles frequently gain new identities and adopt new cellular functions. Autophagosomes, formed in distal axonal compartments need to be transported towards the soma where they fuse with lysosomes to degrade their content. During retrograde trafficking some autophagosomes fuse with late endosomes to form transient organelles called amphisomes, which serve as signaling platforms travelling from one synapse to the other. Similarly, only a fraction of endo-lysosomal organelles in the axon are functional lysosomes and a large number of diverse intermediates have other non-degrative roles. The local availability of degradation-competent lysosomes is likely one of the rate-limiting factors controlling the transformation of autophagosomes into autolysosomes. With this project, we want to understand the processes involved in autophagosomal and lysosomal maturation in axons. We hypothesize that trafficking of autophagosomal and endo-lysosomal organelles, dependent on the coordinated interplay of actin and microtubule motor proteins, is essential for the timing of organelle’s transitions and the location where these transitions might happen. Furthermore, we aim to address unconventional roles of the endo-lysosomal and autophagy systems at the pre-synapse by investigating exocytosis as a mechanism responsible for a rapid clearance of ‘aged’ synaptic components.
References:
Bucher ,M., Niebling, S., Han, Y., Molodenskiy, D., Hassani Nia, F., Kreienkamp, H.J., Svergun, D., Kim, E., Kostyukova, A.S., Kreutz, M.R., Mikhaylova, M. (2021) Autism-associated SHANK3 missense point mutations impact conformational fluctuations and protein turnover at synapses. Elife. 10:e66165.
van Bommel, B., Konietzny, A., Kobler, O., Bär, J., Mikhaylova, M. (2019). F-actin patches associated with glutamatergic synapses control positioning of dendritic lysosomes. EMBO J. 1; 38(15).
Mikhaylova, M.*, Bär, J., van Bommel, B., Schätzle, P., YuanXiang, P., Raman, R., Hradsky, J., Konietzny, A., Loktionov, E.Y., Reddy, P.P., Lopez-Rojas, J., Spilker, C., Kobler, O., Raza, S.A., Stork, O., Hoogenraad, C.C., Kreutz, M.R.* (2018). Caldendrin Directly Couples Postsynaptic Calcium Signals to Actin Remodeling în Dendritic Spines. Neuron.97:1110-1125. *shared correspondence
Mikhaylova, M., Bera, S., Kobler, O., Frischknecht, R., Kreutz, M.R. (2016). A Dendritic Golgi Satellite between ERGIC and Retromer. Cell Rep. 14:189-99.
